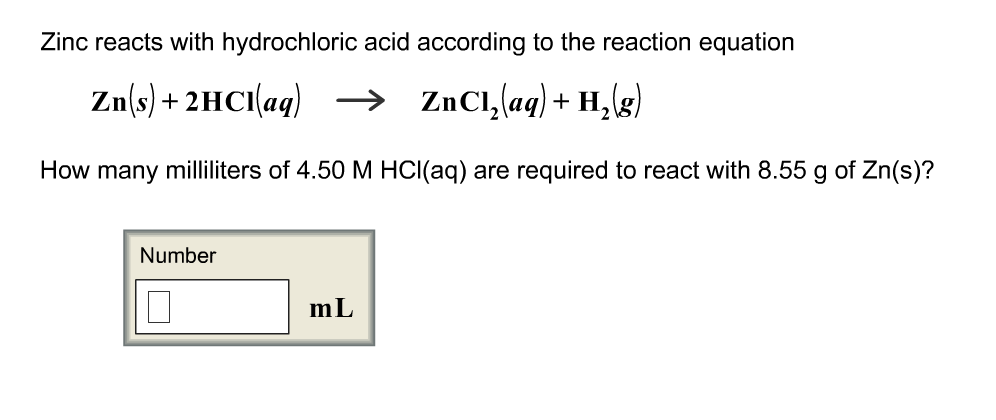
SOLVED: In the reaction Zn + H+ —> Zn2+ + H2, Zn is oxidized and H is reduced. How many electrons would be produced and used in the balanced half-reactions? Complete the
Consider the following reactions (unbalanced) Zn + hot conc. H2SO4 →G + R + X - Sarthaks eConnect | Largest Online Education Community

Question Video: Describing the Correct Symbol Equation for the Reaction between Zinc Metal and Hydrochloric Acid | Nagwa
43. Balance the following equation: Zn + (H+) —> (Zn+2) + H2 (Zinc reacts with hydrogen ion to give Zinc ion and Hydrogen gas.)
![Molecular Zinc Hydride Cations [ZnH]+: Synthesis, Structure, and CO2 Hydrosilylation Catalysis - Ritter - 2020 - Angewandte Chemie International Edition - Wiley Online Library Molecular Zinc Hydride Cations [ZnH]+: Synthesis, Structure, and CO2 Hydrosilylation Catalysis - Ritter - 2020 - Angewandte Chemie International Edition - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/8fb7eef0-0324-42ed-a2b2-135946ab80b4/anie202011480-toc-0001-m.png)
Molecular Zinc Hydride Cations [ZnH]+: Synthesis, Structure, and CO2 Hydrosilylation Catalysis - Ritter - 2020 - Angewandte Chemie International Edition - Wiley Online Library

Treatment of benzaldehyde (C_6H_5CHO) with Zn(Hg) in aqueous HCl forms a compound Z that has a molecular ion at 92 in its mass spectrum. Z shows absorptions at 3150-2950, 1605, and 1496
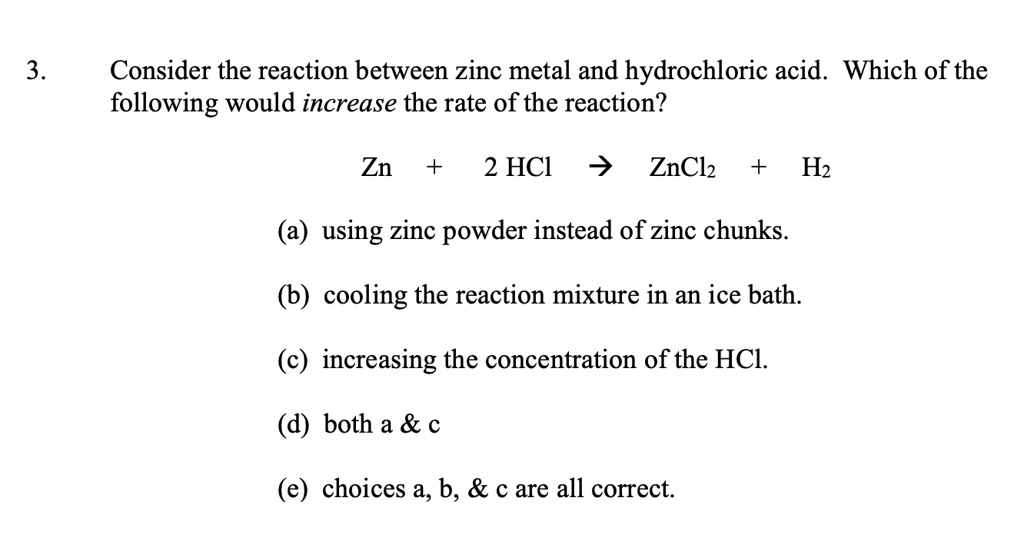
SOLVED: Consider the reaction between zinc metal and hydrochloric acid. Which of the following would increase the rate of the reaction? 3. ZnCl2 2. HCl Zn H2 using zinc powder instead of
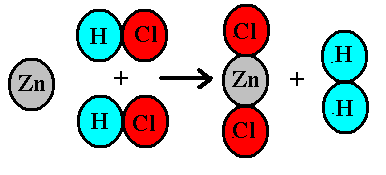
In the reaction Zn + 2HCl -> ZnCl_2 + H_2, how many moles of hydrogen will be formed when 4 moles of HCl are consumed? | Socratic
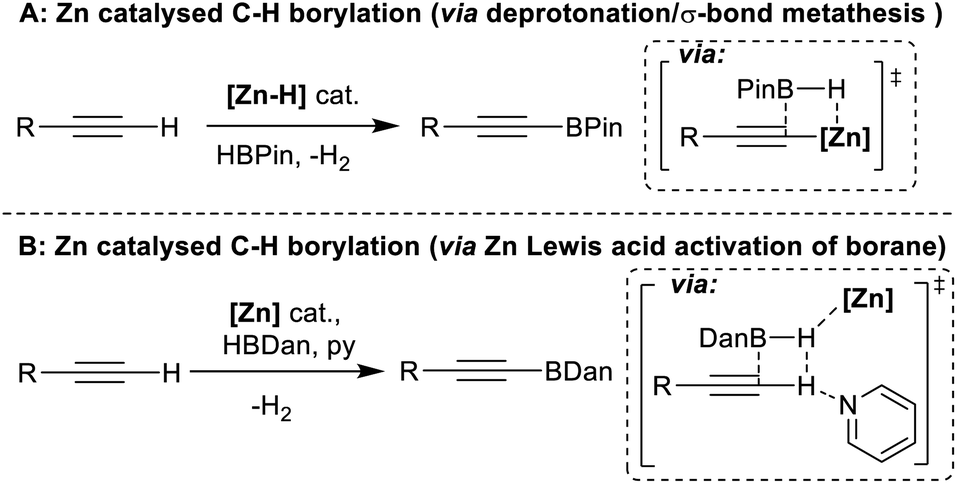
Zinc catalysed electrophilic C–H borylation of heteroarenes - Chemical Science (RSC Publishing) DOI:10.1039/D1SC01883C


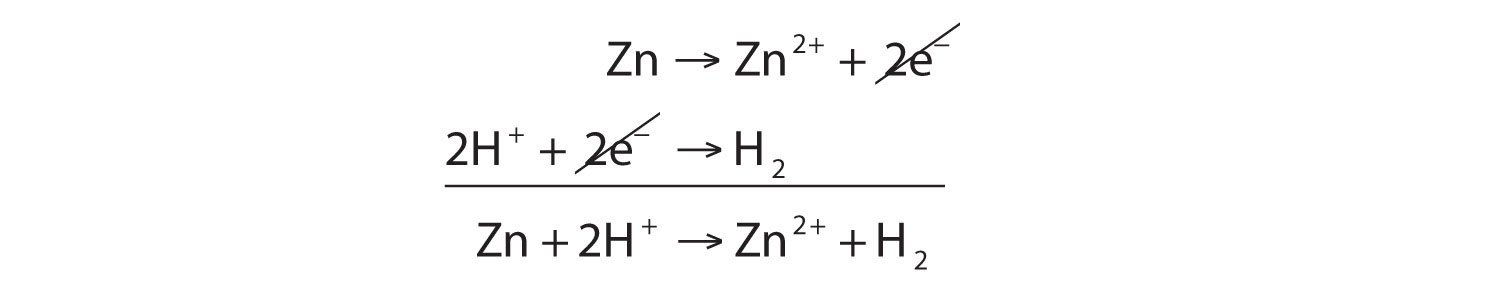
![In the reaction, C6H5COCH3 [Zn - Hg/conc. HCl][H]X . X is: In the reaction, C6H5COCH3 [Zn - Hg/conc. HCl][H]X . X is:](https://dwes9vv9u0550.cloudfront.net/images/7671796/5bdff3d8-c0c9-4fc8-89cd-f9235a0c1b30.jpg)